NIS4®*
A proprietary technology that underlies a novel, blood-based molecular biomarker test to rule in and/or rule out at-risk NASH (NAS ≥ 4 and F ≥ 2) in patients with at least one metabolic risk factor.1
NASH, the most severe form of NAFLD, is a highly underdiagnosed cause of severe liver complications- NAFLD is estimated to affect nearly 80 million people in the US, but only 5% are aware they have liver disease.1
NAS, NAFLD activity score;
NASH, non-alcoholic steatohepatitis;
NAFLD, non-alcoholic fatty liver disease.
NIS4® technology has been licensed to Labcorp for use for medically appropriate patients enrolled in a clinical trial, regardless of the trial sponsor.
Receive real-time information and resources related to NIS4® technology and its future applications.
SIGN UPCertain patients are at an increased risk of developing NASH and liver-related outcomes.2,3
RISK FACTORS4,5
NAFLD/NASH is highly associated with other metabolic co-morbidities, including type 2 diabetes, hypertension, dyslipidemia, and central adiposity.
NAFLD6
An estimated 30% to 40% of the US adult population is currently affected by NAFLD.
NASH7
NASH is a severe form of NAFLD characterized by hepatocyte inflammation and liver cell injury, or ballooning.
NASH AND FIBROSIS8*
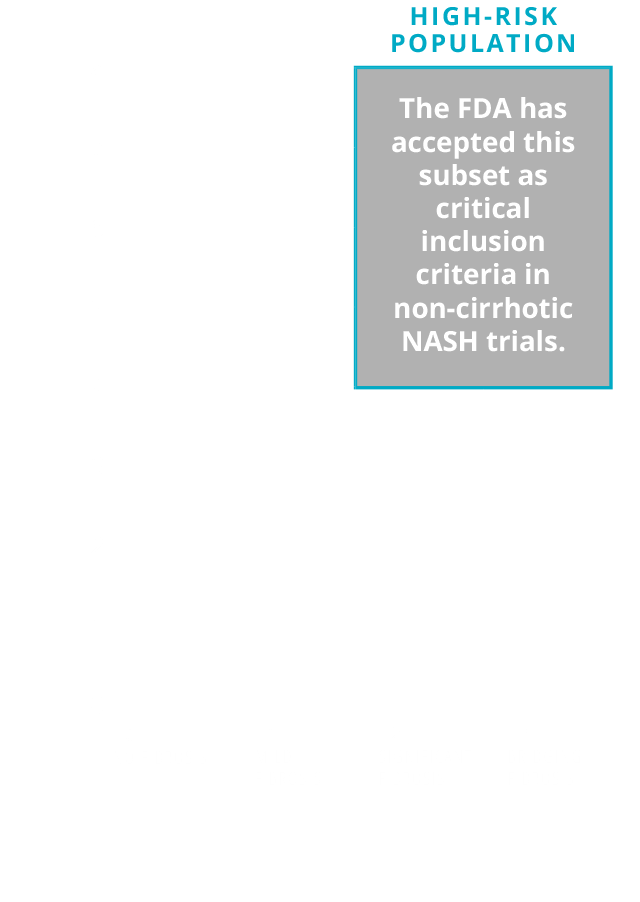
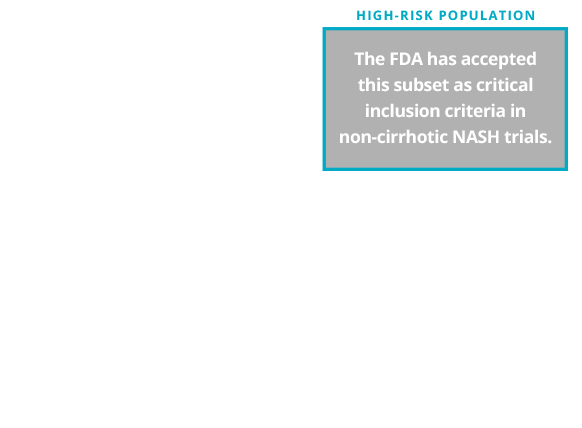
Patients with NASH (NAS ≥4) and significant fibrosis (F≥2) are considered to have a higher likelihood of disease progression and may benefit from future pharmacotherapeutic interventions. As such, this population is often recruited for participation in NASH clinical trials.
OUTCOMES2,3
NASH disease progression may result in cirrhosis, liver failure, and hepatocellular carcinoma (HCC).
RISK FACTORS4,5
NAFLD/NASH is highly associated with other metabolic co-morbidities, including type 2 diabetes, hypertension, dyslipidemia, and central adiposity.
NAFLD6
An estimated 30% to 40% of the US adult population is currently affected by NAFLD.
NASH7
NASH is a severe form of NAFLD characterized by hepatocyte inflammation and liver cell injury, or ballooning.
NASH AND FIBROSIS8*
Patients with NASH (NAS ≥4) and significant fibrosis (F≥2) are considered to have a higher likelihood of disease progression and may benefit from future pharmacotherapeutic interventions. As such, this population is often recruited for participation in NASH clinical trials.
OUTCOMES2,3
NASH disease progression may result in cirrhosis, liver failure, and hepatocellular carcinoma (HCC).
NAFLD, non-alcoholic fatty liver disease.
Severity of NASH is determined using the NAFLD activity score (NAS 0 – NAS 8) and the fibrosis stage (F0 – F4).4
A subset of the overall NASH population is at higher risk of progression to cirrhosis.8
There is an unmet need for diagnostic solutions that assess both NASH activity and fibrosis stage whose clinical performance is robust in subsets of patients who are at higher risk of progression to cirrhosis.8
Limitations across NASH testing modalities may preclude the accurate diagnosis of clinically relevant patient populations.
Our Solution
NIS4® is a proprietary technology that underlies a blood-based molecular diagnostic test for identifying NASH and fibrosis.1
APRI, AST to platelet ratio index; ELF, enhanced liver fibrosis.
NIS4® technology has been licensed to Labcorp for use for medically appropriate patients enrolled in a clinical trial, regardless of the trial sponsor.
Interested in using a NIS4®-powered test in a clinical trial? CONTACT LABCORP DRUG DEVELOPMENTAPRI, AST to platelet ratio index; ELF, enhanced liver fibrosis.
NIS4® technology has been licensed to Labcorp for use for medically appropriate patients enrolled in a clinical trial, regardless of the trial sponsor.
An innovative approach built upon miRNA science.
- NIS4®* assigns a single score, reflecting both NASH activity level and fibrosis stage, to patients based on blood/serum levels of 4 biomarkers: miR-34a-5p, YKL-40, alpha2-macroglobulin, and HbA1c1
- Among all circulating biomarkers tested, miR-34a-5p was the most discriminatory single biomarker for the identification of patients with at-risk NASH (NAS ≥4 and F ≥2)1
NIS4® technology has been licensed to Labcorp for use for medically appropriate patients enrolled in a clinical trial, regardless of the trial sponsor.
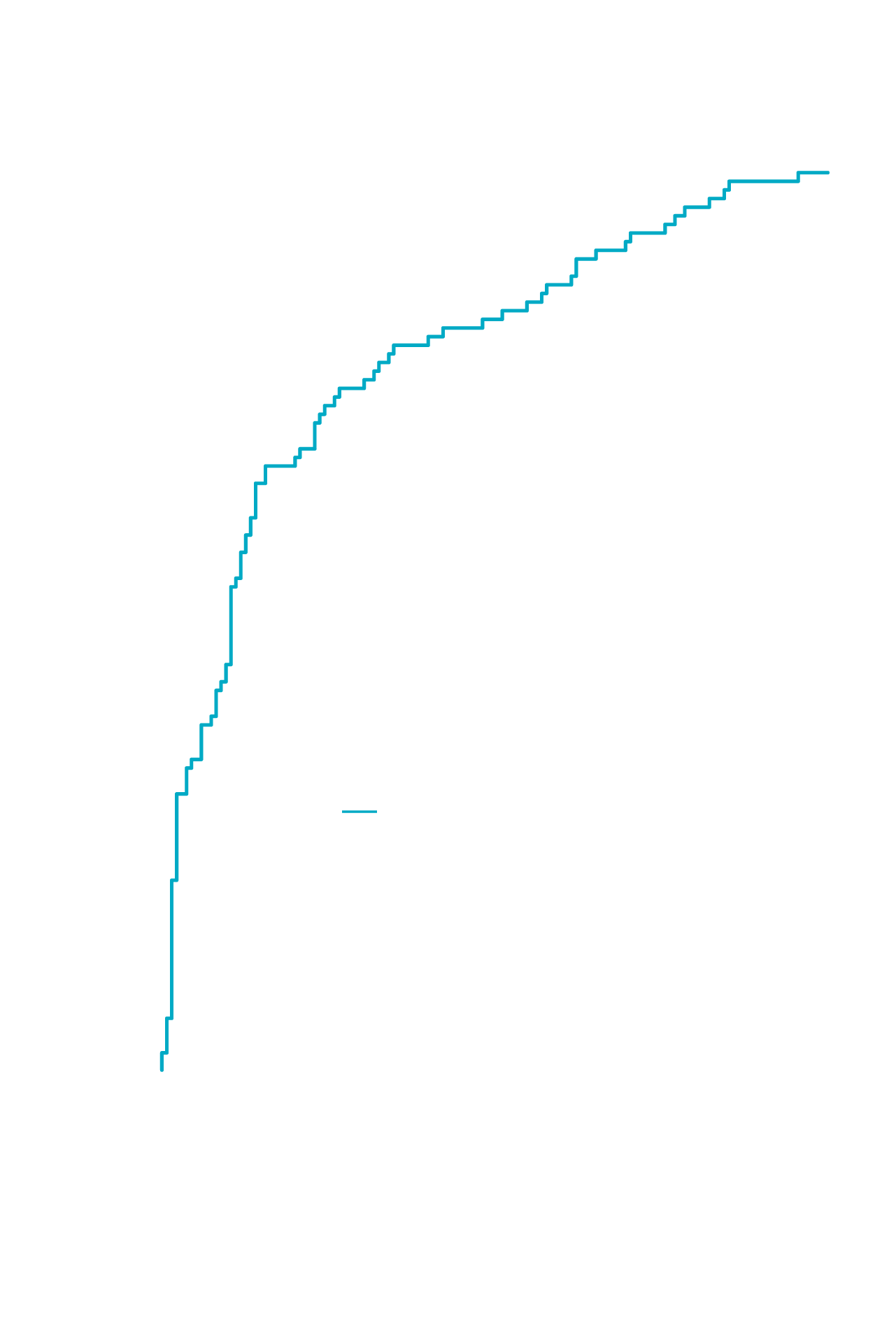
A2M, alpha-2 macroglobulin;
AUROC, area under the receiver operating characteristics curve;
HbA1c, glycated haemoglobin A;
NAS, non-alcoholic fatty liver disease activity score;
NASH, non-alcoholic steatohepatitis.
Reprinted from Lancet Gastroenterol Hepatol, Harrison SA, Ratziu V, Boursier J, et al, A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study, August 4, 2020 [Epub ahead of print], DOI: https://doi.org/10.1016/S2468-1253(20)30252-1, Copyright 2020, with permission from Elsevier.
NIS4®*: designed specifically for patients across the NASH/NAFLD spectrum.
NIS4® is the first and only technology that underlies a novel, blood-based biomarker test intended to identify patients with at-risk NASH (NAS ≥4 and F ≥2).1†
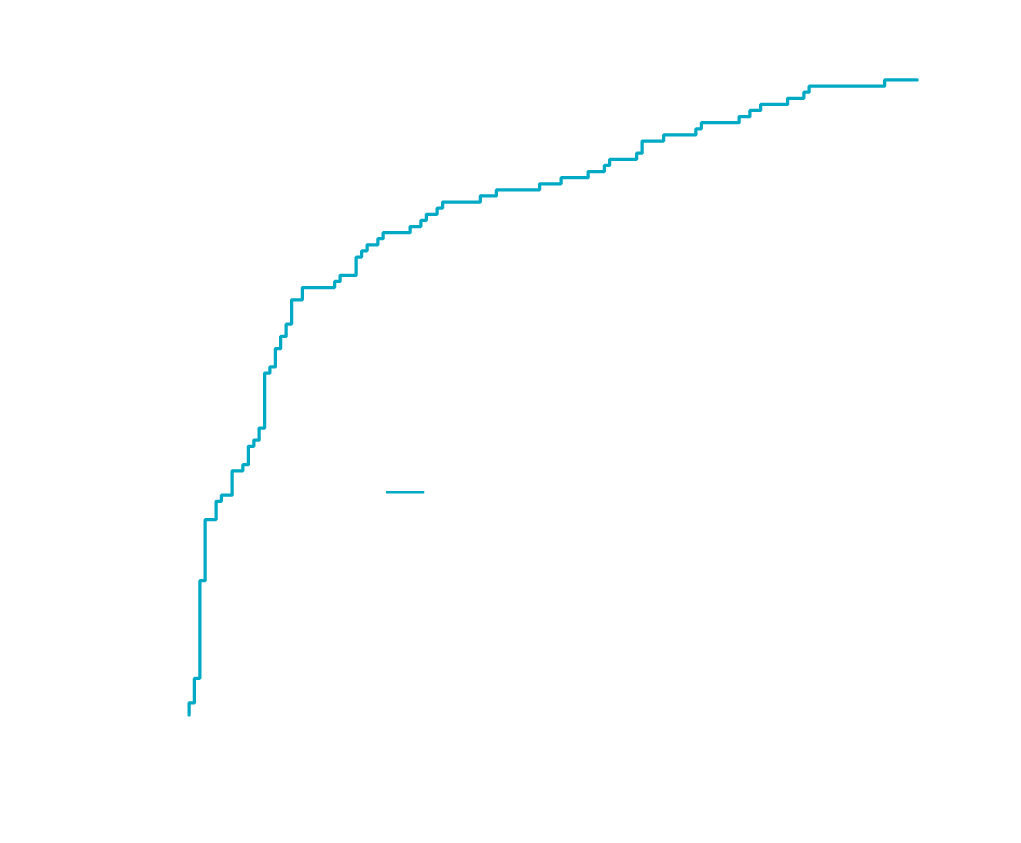
- NIS4® performance data have been generated against liver biopsy in more than 700 patients across the NASH/NAFLD spectrum
- Modeling and validation of clinical performance were formed on independent patient cohorts
- Consistently high diagnostic accuracy was observed irrespective of age, sex, or presence or absence of type 2 diabetes, hypertension, dyslipidemia, BMI, or PNPLA3‡ genotype

Adapted from Lancet Gastroenterol Hepatol, Harrison SA, Ratziu V, Boursier J, et al, A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study, August 4, 2020 [Epub ahead of print], DOI: https://doi.org/10.1016/S2468-1253(20)30252-1, Copyright 2020, with permission from Elsevier.
NIS4® technology has been licensed to Labcorp for use for medically appropriate patients enrolled in a clinical trial, regardless of the trial sponsor.
†Test derivation and validation methods: Clinical data, blood samples, and liver biopsies from patients screened and/or enrolled in two independent prospective trials in NASH, and an independent large retrospective cohort of patients with suspected NAFLD, were used to derive and validate the non-invasive diagnostic tool. In the discovery cohort (n=239), a logistic regression approach derived the 4-biomarker algorithm correlated to biopsy-derived NAFLD activity score ≥4, fibrosis stage ≥2. The non-invasive diagnostic tool was then validated in 2 independent cohorts: RESOLVE-IT diagnostic cohort (n=475) and ANGERS cohort (n=227). AUC was assessed across these validation cohorts, as well as in the pooled validation cohort (n=702) that was deemed representative of the future intended use population. AUC analyses were repeated in subpopulations of patients defined by age, sex, BMI, type 2 diabetes status, PNPLA3 genotype, and the presence or absence of hypertension and dyslipidemia.
‡ Data only available in RESOLVE-IT diag cohort (n=475).
References: 1. Harrison SA, Ratziu V et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol, (in press). Accessed August 3, 2020. https://www.thelancet.com/journals/langas/article/PIIS2468-1253(20)30252-1/fulltext 2. Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5). 3. Francque SM. The role of non-alcoholic fatty liver disease in cardiovascular disease. Eur Cardiol. 2014;9(1):10-15. 4. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005-2023. 5. Bang KB, Cho YK. Comorbidities and Metabolic Derangement of NAFLD. J Lifestyle Med. 2015;5(1):7-13. 6. Definition & facts of NAFLD & NASH. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts. Published 2019. Accessed March 4, 2020. 7. Sanyal AJ, Harrison SA, Ratziu V, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology. 2019;70(6):1913-1927. 8. Center for Drug Evaluation and Research. Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment. US Department of Health and Human Services, Food and Drug Administration; 2018.
Receive real-time information and resources related to NIS4® technology and its future applications.
SIGN UPInterested in using a NIS4®-powered test in a clinical trial? CONTACT LABCORP DRUG DEVELOPMENT Interested in using a NIS4®-powered test in your healthcare practice? CONTACT LABCORP
Click here to access The Lancet Gastroenterology & Hepatology publication on NIS4® technology